The safety features
All prescription medicine packs must contain these safety features:
- Anti-tampering device
- 2D matrix code: The unique information on the 2D matrix code on the pack consists of the unique serial number, the product code, the batch number and the expiry date.
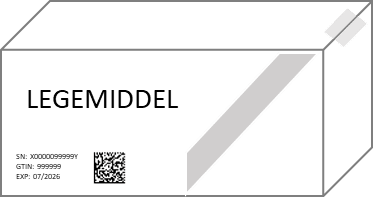
When dispensing prescription medicine, the 2D matrix code is scanned and a verification is done against the national verification system (NMVS). The information contained in the NMVS is uploaded from the central part of the system, which is the EU HUB.
NMVS is a pan-European system with a database for each EU and EEA country. The end users in the various countries are connected to the database in their own country. There are two suppliers for the national systems; Arvato Systems (ARV) and SolidSoft Reply (SSR). Norway has chosen Arvato as our system supplier, together with 16 other countries. These 17 countries work together in a customer group regarding the development, operation and maintenance of the system, together with Arvato. This means that each country can have small organisations, but together you have a strong apparatus with great expertise on the system and on the directive’s regulations.
Suppliers of solutions (SWS) to wholesalers, hospitals and/or pharmacies can develop integration with NMVS NO (the Norwegian database) and thus connect. A supplier can both represent a single end user and/or a group of end users. For the connection process see information on the Software supplier pages.